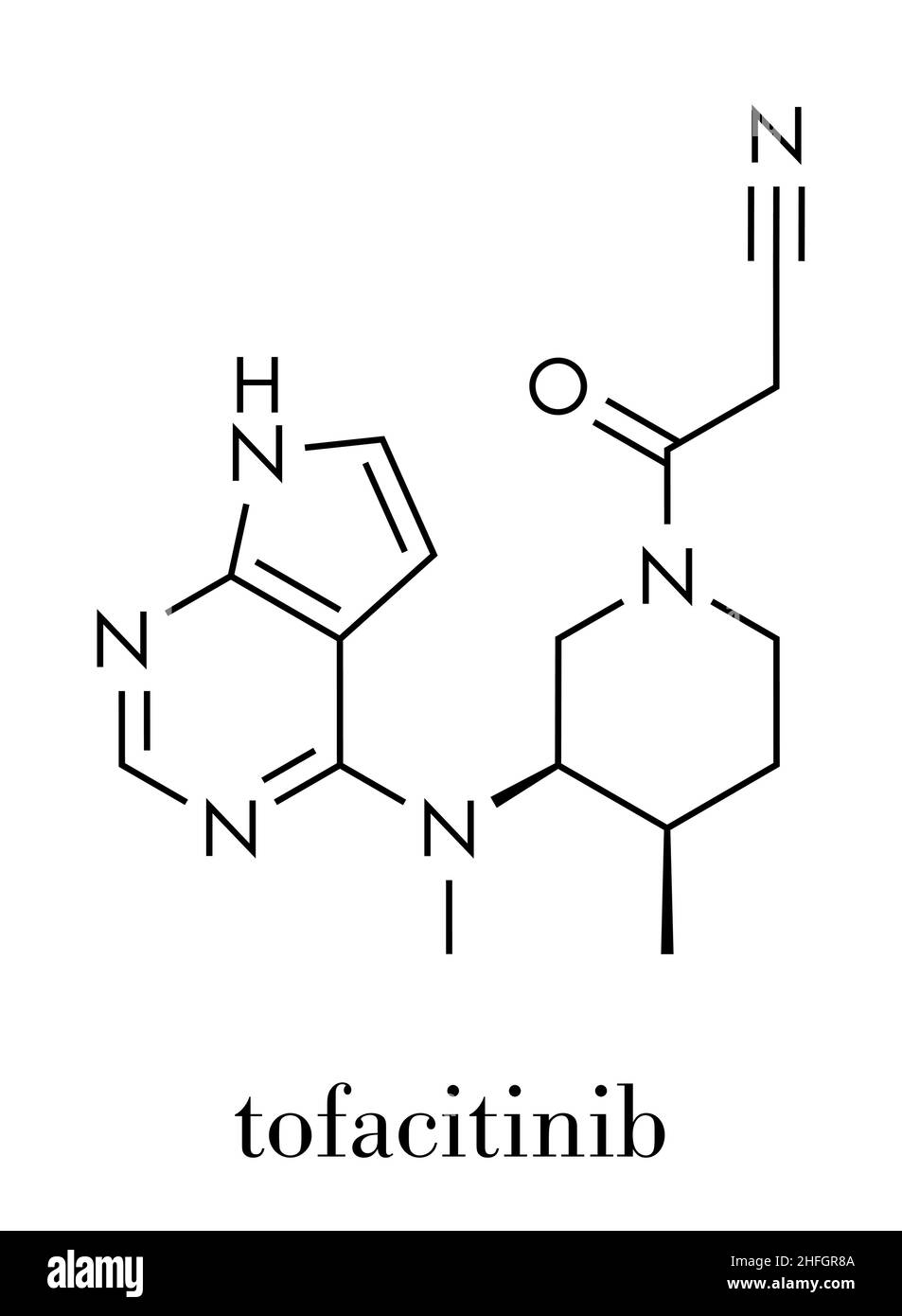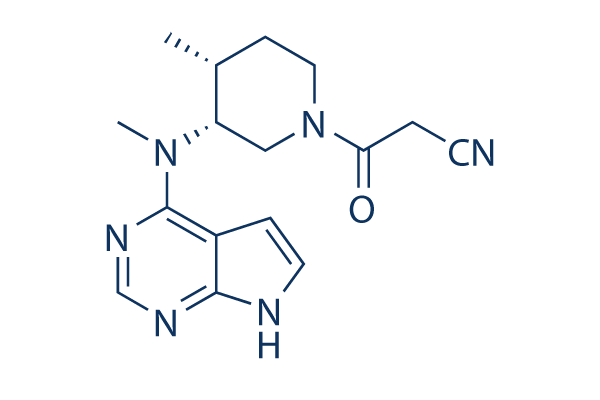
FDA Crisis for Pfizer: The Impact of an FDA Warning On the Company – Cases and Tools in Biotechnology Management

Pfizer and Lilly's JAK inhibitor safety concerns prompt Europe to scrutinize drug class | Fierce Pharma
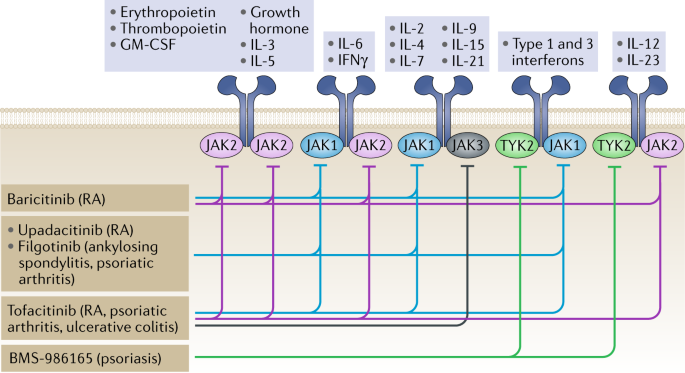
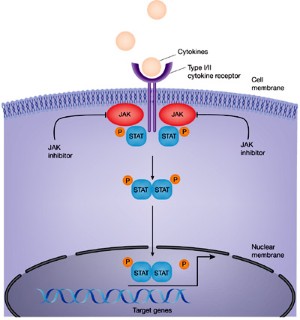
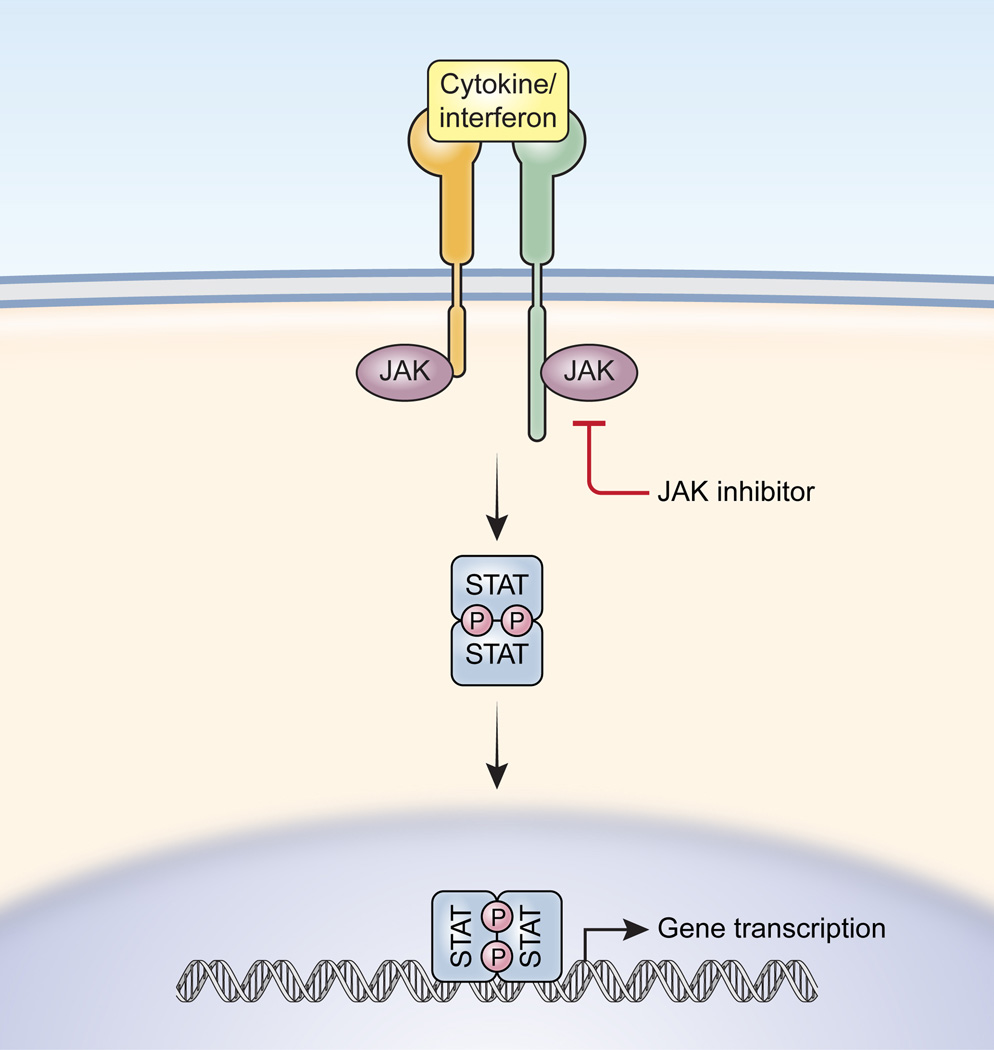
JAK inhibition as a therapeutic strategy for immune and inflammatory diseases | Nature Reviews Drug Discovery

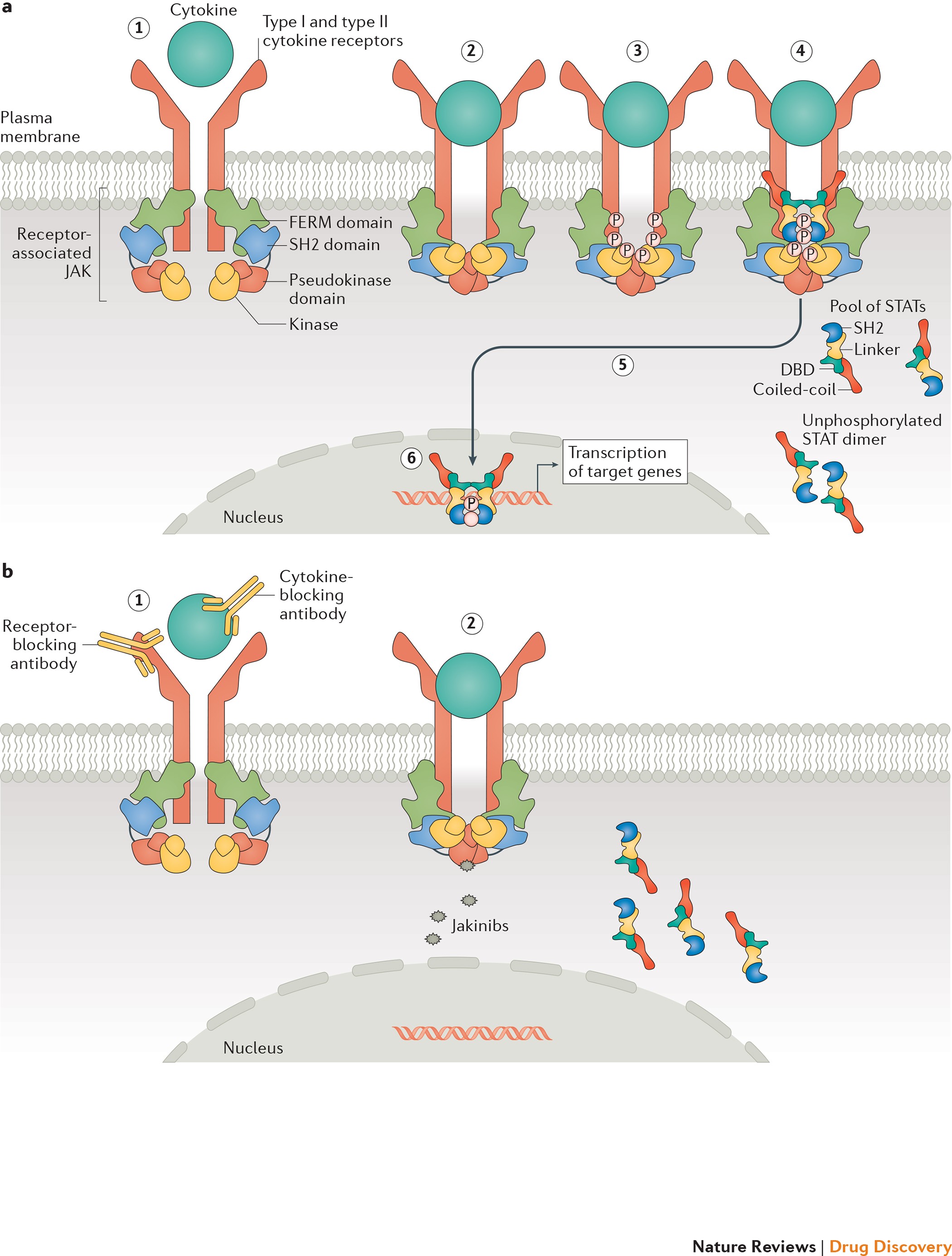
Janus kinase-targeting therapies in rheumatology: a mechanisms-based approach | Nature Reviews Rheumatology
Pfizer Xeljanz, Tofacitinib (tofacitinib citrate), JAK inhibitor, 11mg tablets, once daily treatment in sustained release tablet form, France Stock Photo - Alamy
FDA approves JAK inhibitors as 2nd-line systemic therapy in atopic dermatitis < Pharma < Article - KBR